

Breaking Self-Tolerance: Self-Neoself Discrimination by T Cells in Autoimmunity
Self–Neoself Discrimination by CD4 T Cells: A New Framework for Autoimmunity
Autoimmune diseases are strongly linked to genetic variation in the HLA (MHC) class II region, yet most individuals carrying risk alleles never develop disease. This gap suggests that genetics alone is insufficient and that disease initiation may require a discrete change in antigen presentation. Our work focuses on a conceptual shift from the classical “self versus nonself” model to a “self versus neoself” discrimination model, in which CD4 T cells can respond pathologically to aberrant forms of self generated by altered MHC class II loading pathways.
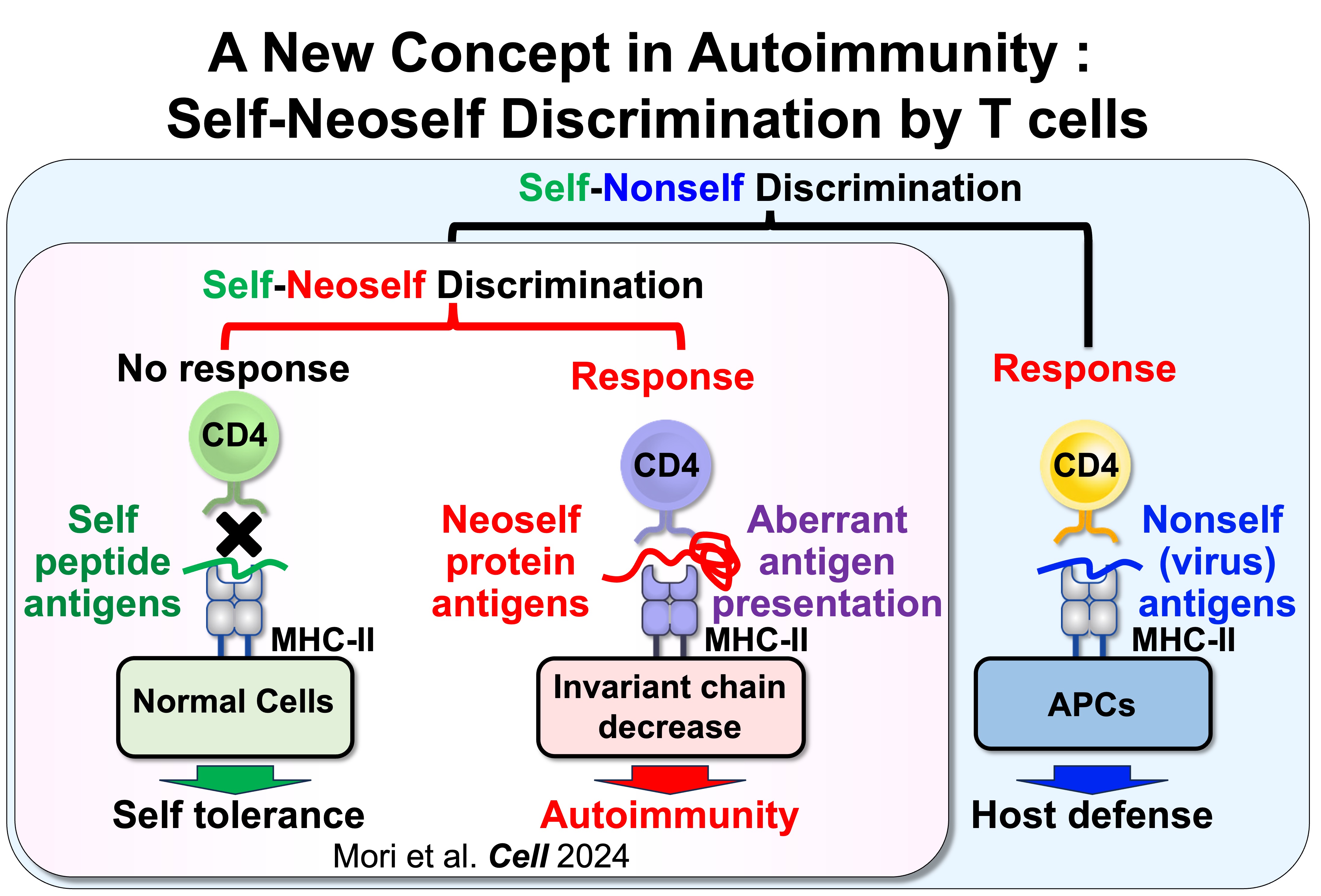
Fig.1 Self-Nonself Discrimination versus Self-Neoself Discrimination
We found that CD4 T cells can distinguish conventional self peptides presented on MHC class II under normal conditions from self antigens that are aberrantly presented on MHC class II when invariant chain expression is low. Because the self antigens presented under these conditions differ markedly from those presented on normal MHC class II, we termed these antigens “neoself antigens.” Notably, induction of neoself-antigen presentation leads to clonal expansion of neoself-reactive T cells and triggers autoimmunity. Moreover, neoself-reactive T cells are clonally expanded in patients with systemic lupus erythematosus. Collectively, these findings suggest that aberrant presentation of neoself antigens plays a critical role in autoimmune disease pathogenesis.
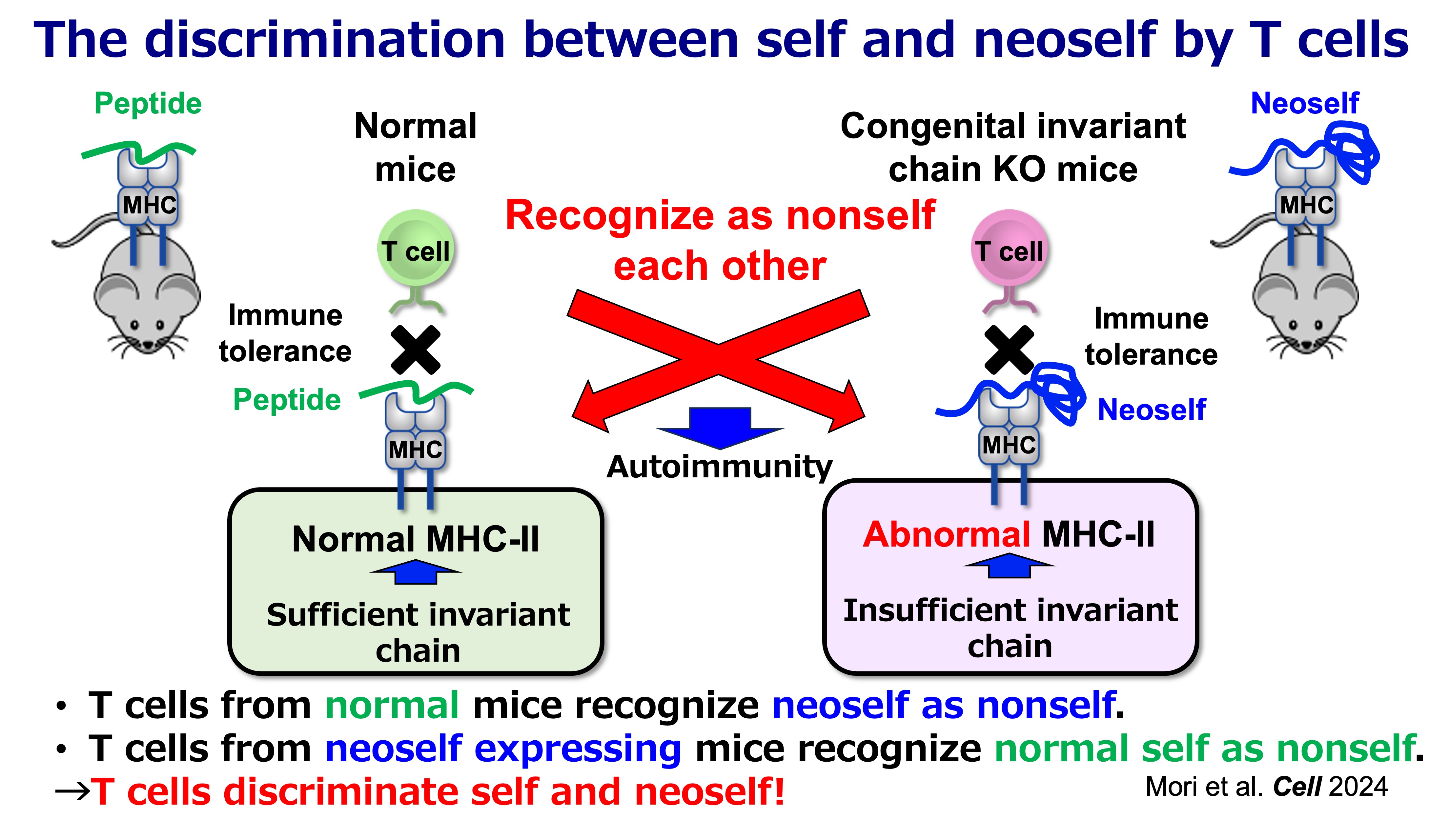
Fig.2 Self-Noeself Discrimination by T cells
What is “neoself” in this context?
In the canonical pathway, MHC class II molecules present peptide fragments produced in endosomal/lysosomal compartments. A central determinant of this pathway is the invariant chain (Ii/CD74), which (i) escorts MHC class II to the appropriate antigen-loading compartments and (ii) protects the peptide-binding groove from inappropriate binding events in the endoplasmic reticulum (ER). In this standard configuration, the repertoire of displayed self is largely “peptidic” and shaped by physiological proteolysis.
When Ii expression is reduced or disrupted, however, MHC class II molecules can engage a qualitatively different substrate: unstructured or partially unfolded regions of endogenous proteins in the ER. These complexes can traffic to the cell surface without the usual endosomal processing steps, resulting in a distinct form of antigen display that we refer to as neoself presentation. Importantly, “neoself” here does not mean mutation-derived neoantigens (as in cancer). Instead, it reflects self molecules acquiring novel immunological meaning because they are presented in an atypical conformational or molecular context.
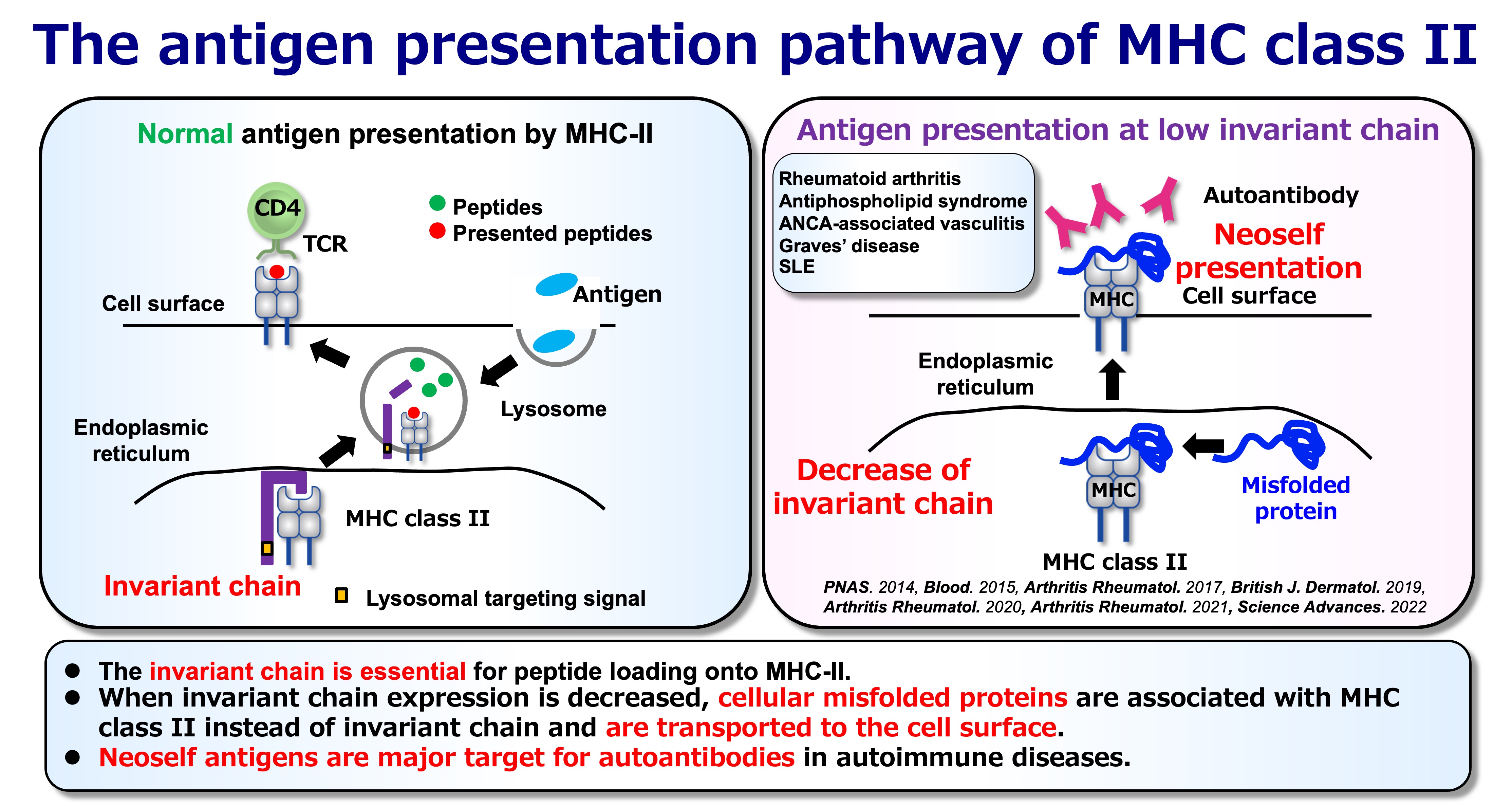
Fig.3 Normal and Abnormal Antigen Presentation
Why neoself antigen presentation reframes immune tolerance
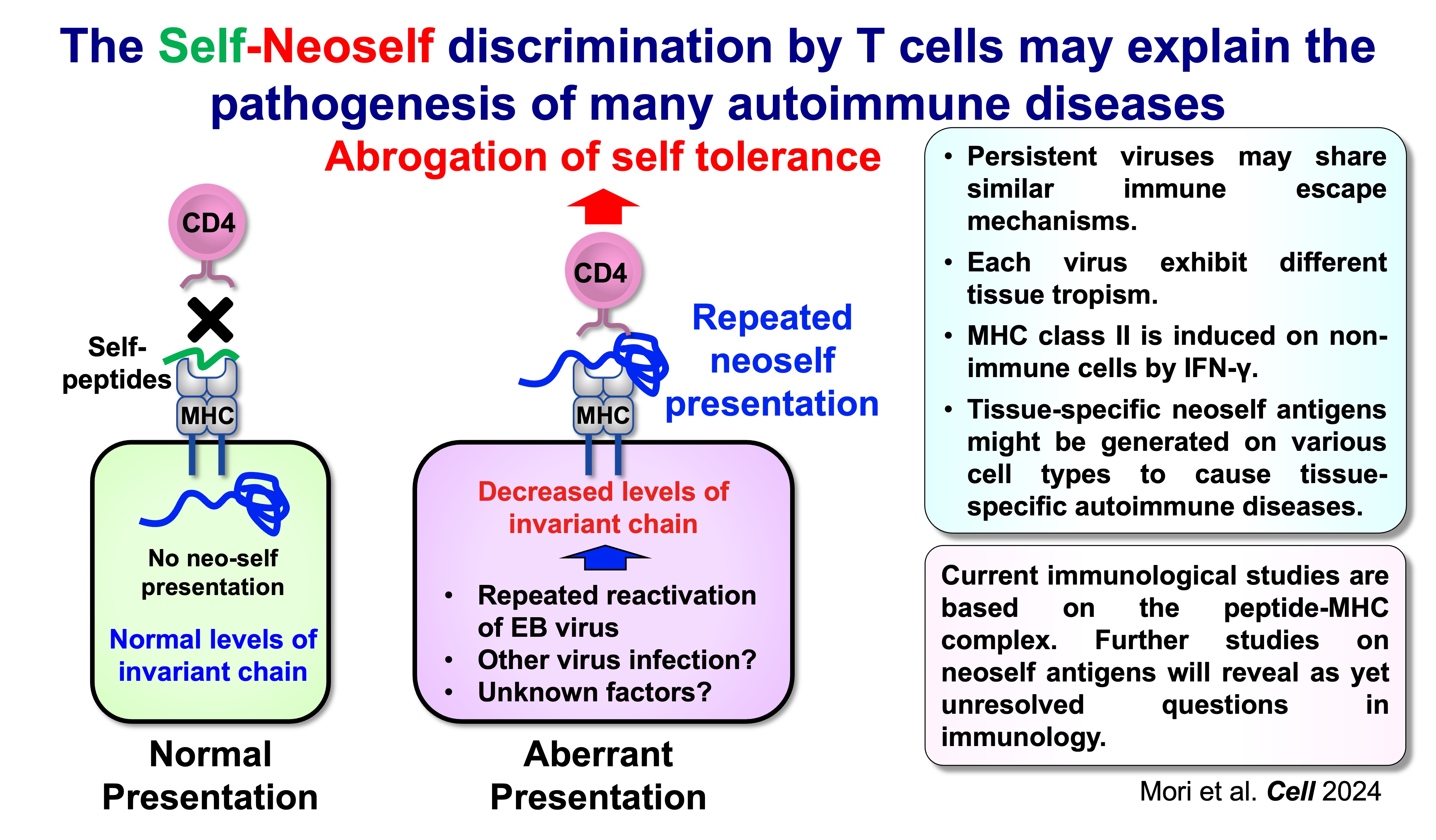
A key implication of neoself presentation is that the immune system’s definition of “self” is not solely determined by molecular identity, but also by how antigens are processed and displayed. If an individual develops under normal conditions (with intact Ii and conventional peptide-centered presentation), T cell tolerance is primarily tuned to that antigenic landscape. A later-life switch to neoself presentation can therefore reveal antigenic structures that were not effectively encountered during tolerance induction, allowing pre-existing T cell clones to interpret neoself as immunologically “foreign.”
This framework is supported by inducible genetic models in which Ii is removed in adulthood. In these settings, emergence of neoself presentation is followed by robust CD4 T cell activation, expansion of T follicular helper (Tfh) populations, germinal center amplification, and broad autoantibody production with immune-complex pathology—features resembling systemic lupus erythematosus (SLE). In contrast, when Ii deficiency is present from early development, the immune system appears better able to incorporate the altered antigen presentation state into tolerance mechanisms. Together, these observations support a simple but consequential proposition: CD4 T cells can discriminate “self” from “neoself,” and that distinction can be decisive for autoimmune initiation.
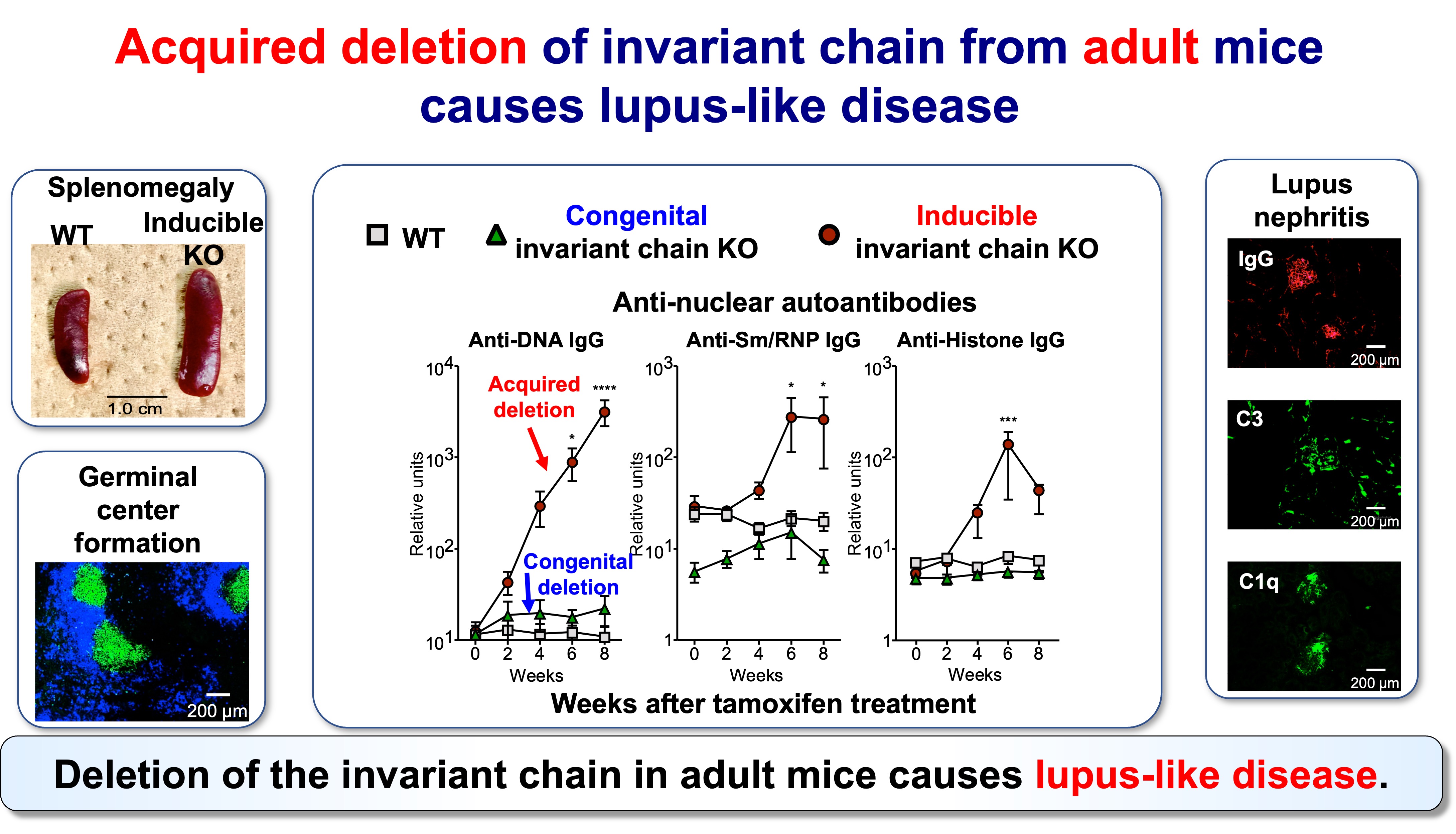
Fig.4 Acquired Invariant Chain KO Mice Develop Lupus-Like Disease
Neoself-reactive CD4 T cells as drivers of lupus-like disease
Neoself presentation provides a mechanistic basis for how CD4 T cells can initiate systemic autoimmunity through B cell help. Expanded CD4 T cell clones can preferentially adopt Tfh-like phenotypes, promoting germinal center reactions and diversification of autoantibody responses. Functional analyses of TCR specificities further indicate that neoself-reactive T cell clones are not incidental; they can contribute directly to autoimmune amplification when present in the appropriate antigen-presentation environment.
This model also aligns with a broader immunological pattern: many disease-associated autoantibodies appear to recognize unusual self configurations, including molecular complexes involving MHC class II. Thus, neoself is best viewed as a category of antigenic “state”—a form of self that becomes visible and immunogenic when antigen loading is perturbed—rather than a single molecule or epitope.
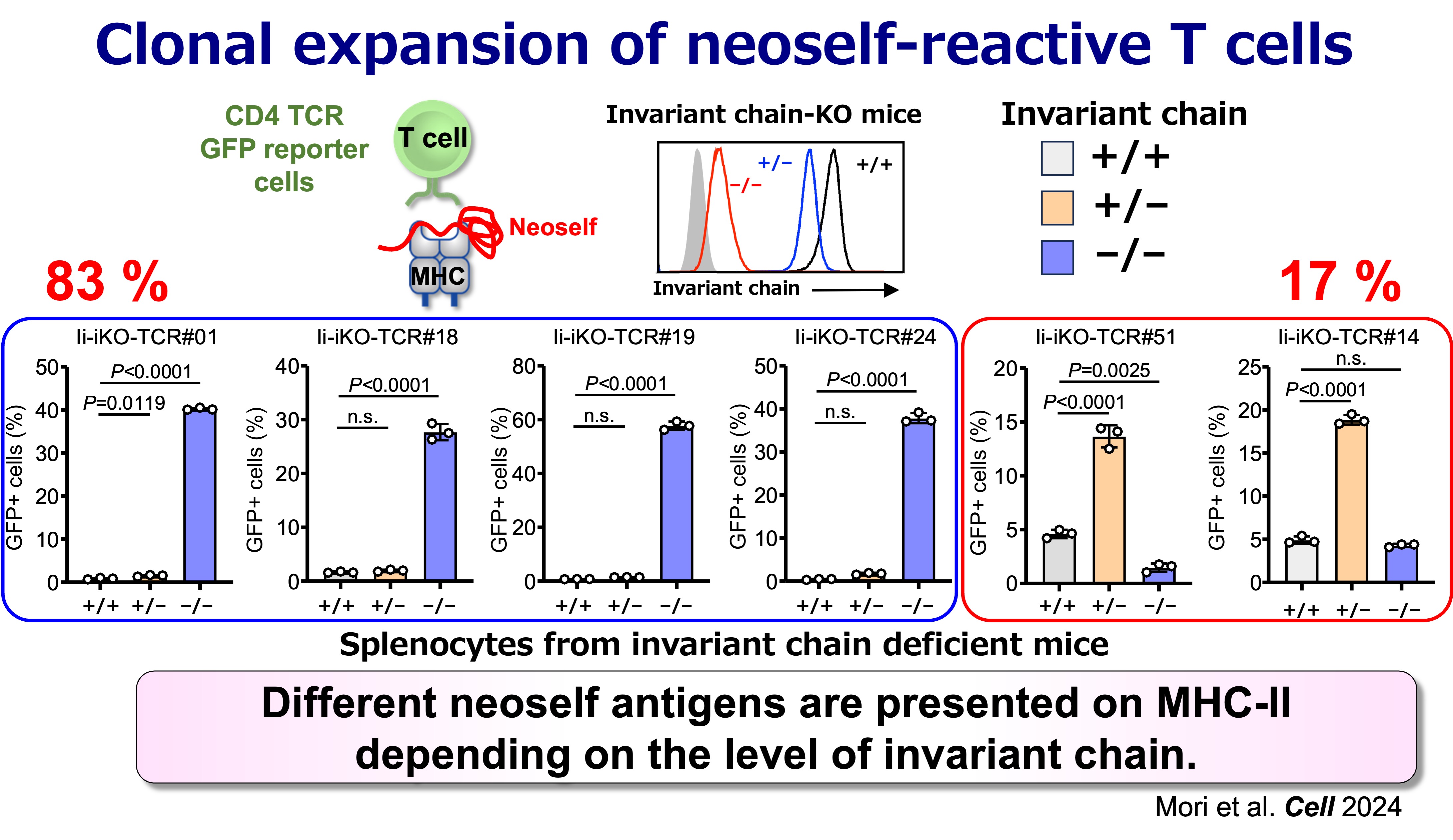
Fig.5 Antigen Specificities of Neoself-Reactive T Cells
Relevance to human SLE
A central translational question is whether neoself-reactive CD4 T cells are expanded in human disease. Evidence from patient-derived TCR analyses supports that clonally expanded CD4 T cells in SLE include substantial neoself-reactive components, consistent with the idea that neoself antigens can represent major immune targets in established disease. This opens a path toward identifying clinically meaningful TCR signatures and antigen-presentation states that may stratify disease mechanisms across patients.
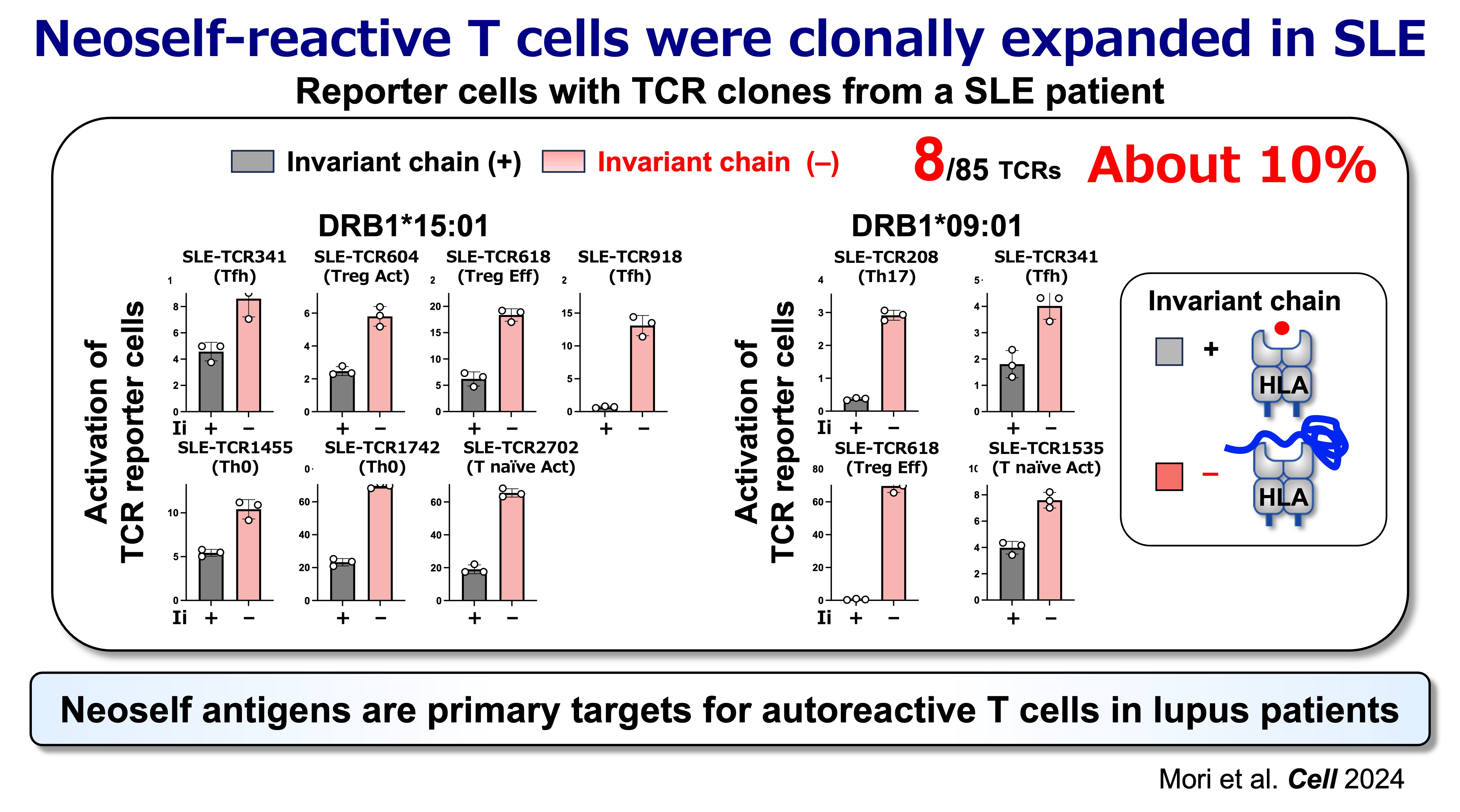
Fig.6 Neoself Antigens are Primary Target for Autoreactive T Cells in Lupus Patients
Environmental triggers: linking persistent viral infection to antigen-presentation shifts
Autoimmunity often follows environmental perturbations, and persistent herpesviruses are recurrent candidates in SLE pathogenesis. A particularly compelling link is Epstein–Barr virus (EBV) reactivation, which can downregulate components of the MHC class II pathway, including Ii, and thereby reshape antigen display toward neoself. In this view, viral immune evasion is not merely a correlate of autoimmunity risk: it can directly induce the antigen-presentation state that allows neoself-reactive CD4 T cells to emerge and expand.
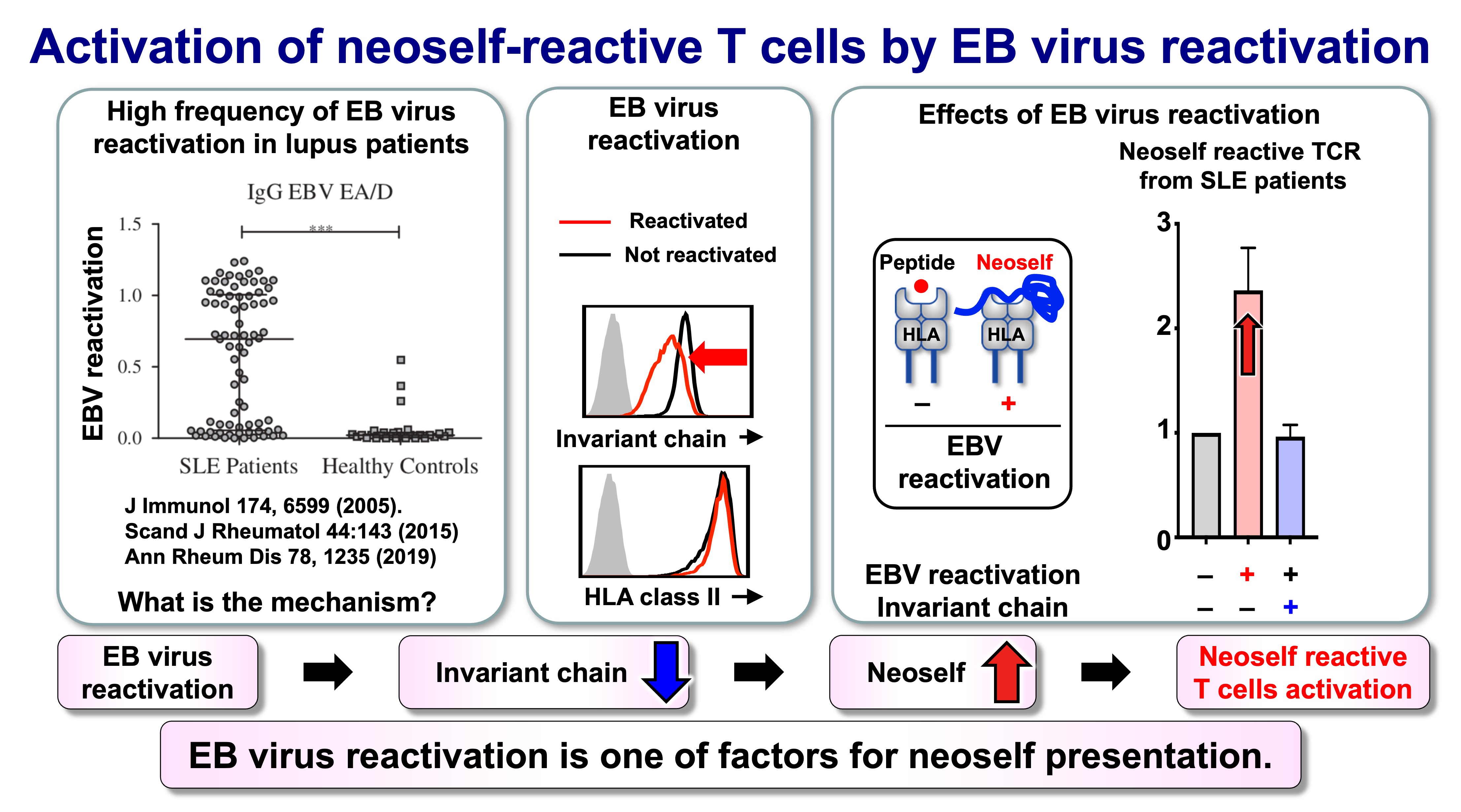
Fig.7 Activation of neoself-reactive T cells by EB virus reactivation
Outlook: toward mechanism-informed diagnostics and intervention
The self–neoself discrimination model suggests new opportunities for both mechanistic and translational advances. On the basic side, we aim to define (i) which antigen-presenting cell types most effectively generate neoself, (ii) how risk HLA class II alleles influence neoself binding and trafficking, and (iii) which TCR features predict pathogenic neoself recognition. On the translational side, neoself-reactive TCRs and markers of altered MHC class II loading may become diagnostic indicators, while restoring or stabilizing canonical antigen-loading pathways could represent a selective therapeutic strategy that targets disease initiation mechanisms without broadly suppressing immunity.
Related publications
Mori S, Kohyama M, Yasumizu Y, Tada A, Tanzawa K, Shishido T, Kishida K, Jin H, Nishide M, Kawada S, Motooka D, Okuzaki D, Naito R, Nakai W, Kanda T, Murata T, Terao C, Ohmura K, Arase N, Kurosaki T, Fujimoto M, Suenaga T, Kumanogo A, Sakaguchi S, Ogawa Y, Arase H
Neoself-antigens are the primary target for autoreactive T cells in human lupus
Cell 2024 187: 6071-6087 [Link] [AI Podcast]
Jin H, Kishida K, Arase N, Matsuoka S, Nakai W, Kohyama M, Suenaga T, Yamamoto K, Sasazuki T, Arase H
Abrogation of self-tolerance by misfolded self-antigens complexed with MHC class II molecules.
Science Advances 2022. 8:eabj9867 [Link]
Tsuji, H, Ohmura, K, Jin, H, Naito, R, Arase, N, Kohyama, K, Suenaga, T, Sakakibara, S, Kochi, Y, Okada, Y, Yamamoto, K, Kikutani, H, Morinobu, A, Mimori, T, Arase H
Anti-dsDNA antibodies recognize DNA presented on HLA class II molecules of systemic lupus erythematosus risk alleles.
Arthritis Rheumatol 2022 74:105-111.[Link]
Hiwa R, Ohmura K, Arase N, Jin H, Hirayasu K, Kohyama M, Suenaga T, Saito F , Terao C, Atsumi T, Iwatani H, Mimori T, Arase H.
Myeloperoxidase/HLA class II complexes recognized by autoantibodies in microscopic polyangiitis.
Arthritis Rheumatol. 2017 69:2069-2080. [Link]
Tanimura K, Jin, H, Suenaga T, Morikami S, Arase N, Kishida K, Hirayasu K, Kohyama, M. , Ebina Y, Yasuda S, Horita T, Takasugi K, Ohmura K, Yamamoto K, Katayama I, Sasazuki T, Lanier LL, Atsumi T, Yamada, H. Arase H 2015. β2-glycoprotein I / HLA class II complexes are novel autoantigens in antiphospholipid syndrome.
Blood. 125: 2835-2844. [Link]
Jin H, Arase N, Hirayasu K, Kohyama M, Suenaga T, Saito F, Tanimura K, Matsuoka, S. , Ebina K, Shi K, Toyama-Sorimachi N, Yasuda S, Horita T, Hiwa R, Takasugi K, Ohmura K, Yoshikawa H, Saito T, Atsumi T, Sasazuki T, Katayama I, Lanier LL, Arase H 2014. Autoantibodies to IgG/HLA class II complexes are associated with rheumatoid arthritis susceptibility.
Proc. Natl. Acad. Sci. USA. 111: 3787-3792. [Link]
Jiang Y, Arase N, Kohyama M, Hirayasu K, Suenaga T, Jin H, Matsumoto M, Shida K, L. Lanier LL, Saito T, Arase H 2013. Transport of misfolded endoplasmic reticulum proteins to the cell surface by MHC class II molecules.
Int. Immunol. 25:235-246. [Link]


Breaking Self-Tolerance: Self-Neoself Discrimination by T Cells in Autoimmunity
Self–Neoself Discrimination by CD4 T Cells: A New Framework for Autoimmunity
Autoimmune diseases are strongly linked to genetic variation in the HLA (MHC) class II region, yet most individuals carrying risk alleles never develop disease. This gap suggests that genetics alone is insufficient and that disease initiation may require a discrete change in antigen presentation. Our work focuses on a conceptual shift from the classical “self versus nonself” model to a “self versus neoself” discrimination model, in which CD4 T cells can respond pathologically to aberrant forms of self generated by altered MHC class II loading pathways.

Fig.1 Self-Nonself Discrimination versus Self-Neoself Discrimination
We found that CD4 T cells can distinguish conventional self peptides presented on MHC class II under normal conditions from self antigens that are aberrantly presented on MHC class II when invariant chain expression is low. Because the self antigens presented under these conditions differ markedly from those presented on normal MHC class II, we termed these antigens “neoself antigens.” Notably, induction of neoself-antigen presentation leads to clonal expansion of neoself-reactive T cells and triggers autoimmunity. Moreover, neoself-reactive T cells are clonally expanded in patients with systemic lupus erythematosus. Collectively, these findings suggest that aberrant presentation of neoself antigens plays a critical role in autoimmune disease pathogenesis.

Fig.2 Self-Noeself Discrimination by T cells
What is “neoself” in this context?
In the canonical pathway, MHC class II molecules present peptide fragments produced in endosomal/lysosomal compartments. A central determinant of this pathway is the invariant chain (Ii/CD74), which (i) escorts MHC class II to the appropriate antigen-loading compartments and (ii) protects the peptide-binding groove from inappropriate binding events in the endoplasmic reticulum (ER). In this standard configuration, the repertoire of displayed self is largely “peptidic” and shaped by physiological proteolysis.
When Ii expression is reduced or disrupted, however, MHC class II molecules can engage a qualitatively different substrate: unstructured or partially unfolded regions of endogenous proteins in the ER. These complexes can traffic to the cell surface without the usual endosomal processing steps, resulting in a distinct form of antigen display that we refer to as neoself presentation. Importantly, “neoself” here does not mean mutation-derived neoantigens (as in cancer). Instead, it reflects self molecules acquiring novel immunological meaning because they are presented in an atypical conformational or molecular context.

Fig.3 Normal and Abnormal Antigen Presentation
Why neoself antigen presentation reframes immune tolerance
A key implication of neoself presentation is that the immune system’s definition of “self” is not solely determined by molecular identity, but also by how antigens are processed and displayed. If an individual develops under normal conditions (with intact Ii and conventional peptide-centered presentation), T cell tolerance is primarily tuned to that antigenic landscape. A later-life switch to neoself presentation can therefore reveal antigenic structures that were not effectively encountered during tolerance induction, allowing pre-existing T cell clones to interpret neoself as immunologically “foreign.”
This framework is supported by inducible genetic models in which Ii is removed in adulthood. In these settings, emergence of neoself presentation is followed by robust CD4 T cell activation, expansion of T follicular helper (Tfh) populations, germinal center amplification, and broad autoantibody production with immune-complex pathology—features resembling systemic lupus erythematosus (SLE). In contrast, when Ii deficiency is present from early development, the immune system appears better able to incorporate the altered antigen presentation state into tolerance mechanisms. Together, these observations support a simple but consequential proposition: CD4 T cells can discriminate “self” from “neoself,” and that distinction can be decisive for autoimmune initiation.

Fig.4 Acquired Invariant Chain KO Mice Develop Lupus-Like Disease
Neoself-reactive CD4 T cells as drivers of lupus-like disease
Neoself presentation provides a mechanistic basis for how CD4 T cells can initiate systemic autoimmunity through B cell help. Expanded CD4 T cell clones can preferentially adopt Tfh-like phenotypes, promoting germinal center reactions and diversification of autoantibody responses. Functional analyses of TCR specificities further indicate that neoself-reactive T cell clones are not incidental; they can contribute directly to autoimmune amplification when present in the appropriate antigen-presentation environment.
This model also aligns with a broader immunological pattern: many disease-associated autoantibodies appear to recognize unusual self configurations, including molecular complexes involving MHC class II. Thus, neoself is best viewed as a category of antigenic “state”—a form of self that becomes visible and immunogenic when antigen loading is perturbed—rather than a single molecule or epitope.

Fig.5 Antigen Specificities of Neoself-Reactive T Cells
Relevance to human SLE
A central translational question is whether neoself-reactive CD4 T cells are expanded in human disease. Evidence from patient-derived TCR analyses supports that clonally expanded CD4 T cells in SLE include substantial neoself-reactive components, consistent with the idea that neoself antigens can represent major immune targets in established disease. This opens a path toward identifying clinically meaningful TCR signatures and antigen-presentation states that may stratify disease mechanisms across patients.

Fig.6 Neoself Antigens are Primary Target for Autoreactive T Cells in Lupus Patients
Environmental triggers: linking persistent viral infection to antigen-presentation shifts
Autoimmunity often follows environmental perturbations, and persistent herpesviruses are recurrent candidates in SLE pathogenesis. A particularly compelling link is Epstein–Barr virus (EBV) reactivation, which can downregulate components of the MHC class II pathway, including Ii, and thereby reshape antigen display toward neoself. In this view, viral immune evasion is not merely a correlate of autoimmunity risk: it can directly induce the antigen-presentation state that allows neoself-reactive CD4 T cells to emerge and expand.

Fig.7 Activation of neoself-reactive T cells by EB virus reactivation
Outlook: toward mechanism-informed diagnostics and intervention
The self–neoself discrimination model suggests new opportunities for both mechanistic and translational advances. On the basic side, we aim to define (i) which antigen-presenting cell types most effectively generate neoself, (ii) how risk HLA class II alleles influence neoself binding and trafficking, and (iii) which TCR features predict pathogenic neoself recognition. On the translational side, neoself-reactive TCRs and markers of altered MHC class II loading may become diagnostic indicators, while restoring or stabilizing canonical antigen-loading pathways could represent a selective therapeutic strategy that targets disease initiation mechanisms without broadly suppressing immunity.

Related publications
Mori S, Kohyama M, Yasumizu Y, Tada A, Tanzawa K, Shishido T, Kishida K, Jin H, Nishide M, Kawada S, Motooka D, Okuzaki D, Naito R, Nakai W, Kanda T, Murata T, Terao C, Ohmura K, Arase N, Kurosaki T, Fujimoto M, Suenaga T, Kumanogo A, Sakaguchi S, Ogawa Y, Arase H
Neoself-antigens are the primary target for autoreactive T cells in human lupus
Cell 2024 187: 6071-6087 [Link] [AI Podcast]
Jin H, Kishida K, Arase N, Matsuoka S, Nakai W, Kohyama M, Suenaga T, Yamamoto K, Sasazuki T, Arase H
Abrogation of self-tolerance by misfolded self-antigens complexed with MHC class II molecules.
Science Advances 2022. 8:eabj9867 [Link]
Tsuji, H, Ohmura, K, Jin, H, Naito, R, Arase, N, Kohyama, K, Suenaga, T, Sakakibara, S, Kochi, Y, Okada, Y, Yamamoto, K, Kikutani, H, Morinobu, A, Mimori, T, Arase H
Anti-dsDNA antibodies recognize DNA presented on HLA class II molecules of systemic lupus erythematosus risk alleles.
Arthritis Rheumatol 2022 74:105-111.[Link]
Hiwa R, Ohmura K, Arase N, Jin H, Hirayasu K, Kohyama M, Suenaga T, Saito F , Terao C, Atsumi T, Iwatani H, Mimori T, Arase H.
Myeloperoxidase/HLA class II complexes recognized by autoantibodies in microscopic polyangiitis.
Arthritis Rheumatol. 2017 69:2069-2080. [Link]
Tanimura K, Jin, H, Suenaga T, Morikami S, Arase N, Kishida K, Hirayasu K, Kohyama, M. , Ebina Y, Yasuda S, Horita T, Takasugi K, Ohmura K, Yamamoto K, Katayama I, Sasazuki T, Lanier LL, Atsumi T, Yamada, H. Arase H 2015. β2-glycoprotein I / HLA class II complexes are novel autoantigens in antiphospholipid syndrome.
Blood. 125: 2835-2844. [Link]
Jin H, Arase N, Hirayasu K, Kohyama M, Suenaga T, Saito F, Tanimura K, Matsuoka, S. , Ebina K, Shi K, Toyama-Sorimachi N, Yasuda S, Horita T, Hiwa R, Takasugi K, Ohmura K, Yoshikawa H, Saito T, Atsumi T, Sasazuki T, Katayama I, Lanier LL, Arase H 2014. Autoantibodies to IgG/HLA class II complexes are associated with rheumatoid arthritis susceptibility.
Proc. Natl. Acad. Sci. USA. 111: 3787-3792. [Link]
Jiang Y, Arase N, Kohyama M, Hirayasu K, Suenaga T, Jin H, Matsumoto M, Shida K, L. Lanier LL, Saito T, Arase H 2013. Transport of misfolded endoplasmic reticulum proteins to the cell surface by MHC class II molecules.
Int. Immunol. 25:235-246. [Link]